Chitosan: A Scaffold Biomaterial in 3D Bone Tissue Engineering and Its Biological Activities
-
Abstract:
The ability to replicate the microenvironment of the human body through the fabrication of scaffolds is a significant achievement in the biomedical field. However, the search for the ideal scaffold is still in its infancy and there are significant challenges to overcome. In the modern era, the scientific community is increasingly turned to natural substances due to their superior biological ability, lower cost, biodegradability, and lower toxicity than synthetic lab-made products. Chitosan is a well-known polysaccharide that has recently garnered a high amount of attention for its biological activities, especially in 3D bone tissue engineering. Chitosan closely matches the native tissues and thus stands out as a popular candidate for bioprinting. This review focuses on the potential of chitosan-based scaffolds for advancements and the drawbacks in bone treatment. Chitosan-based nanocomposites have exhibited strong mechanical strength, water-trapping ability, cellular interaction, and biodegradability. Chitosan derivatives have also encouraged and provided different routes for treatment and enhanced biological activities. 3D tailored bioprinting has opened new doors for designing and manufacturing scaffolds with biological, mechanical, and topographical properties.
-
Keywords:
- chitosan /
- 3D bioprinting /
- bone tissue engineering /
- scaffold /
- tissue regeneration /
- chitosan derivative
摘要:通过制造支架来模拟人体微环境是生物医学领域的一大成就。然而,寻找理想生物支架的工作仍处于起步阶段,需要克服重大挑战。目前,科学研究更倾向于天然物质,因为它们具有极强的生物能力、低成本和生物可降解性,并且比合成实验室制造的产品毒性更小。壳聚糖是一种著名的多糖,因其生物活性而备受关注,尤其是在 3D 骨组织工程中。壳聚糖与天然组织非常相似,因此是生物打印的热门候选材料。本文重点分析了基于壳聚糖支架发展的潜力以及骨治疗的缺点。壳聚糖纳米复合材料具有较强的机械强度、吸水能力、细胞相互作用和生物降解特性。壳聚糖衍生物还提供了不同的治疗途径,并且具有较强的生物活性。 3D 定制生物打印为设计和制造具有生物、机械和地形特性的支架打开了新的大门。
-
1. Introduction
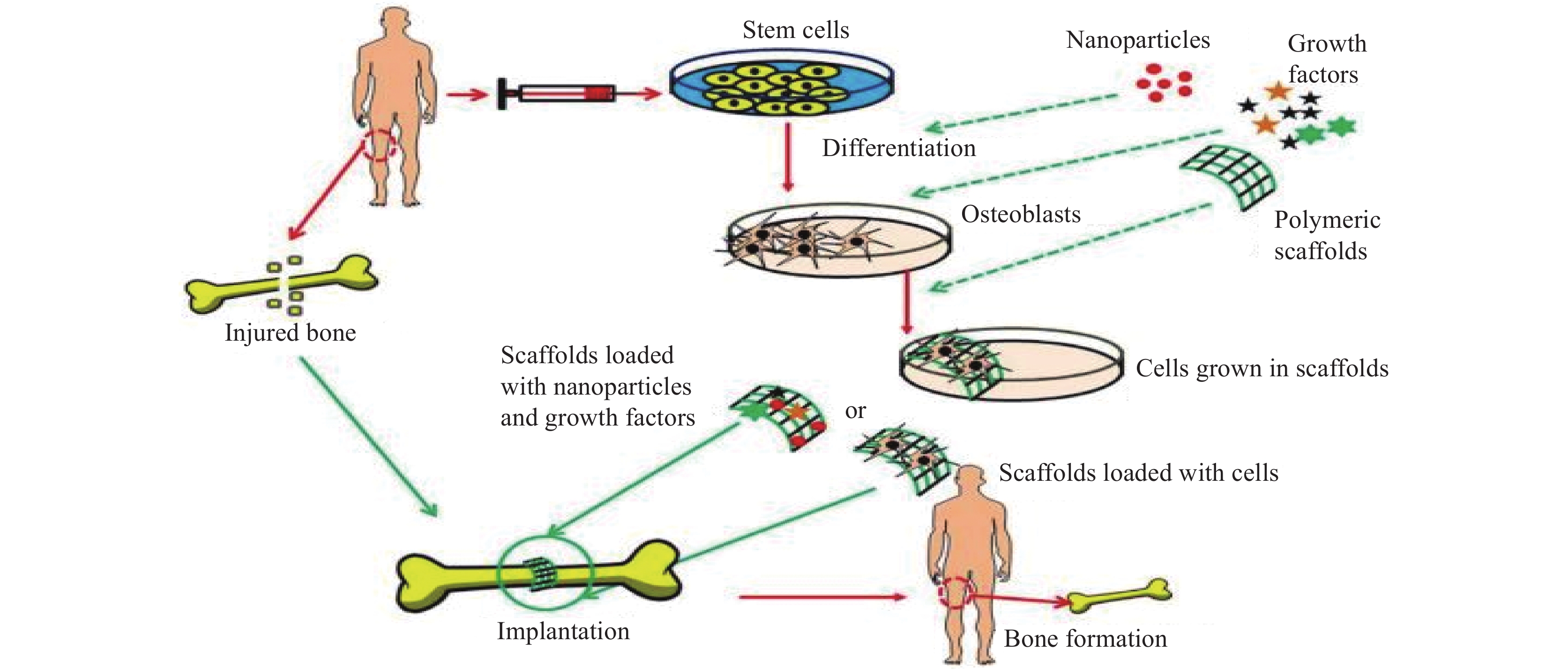
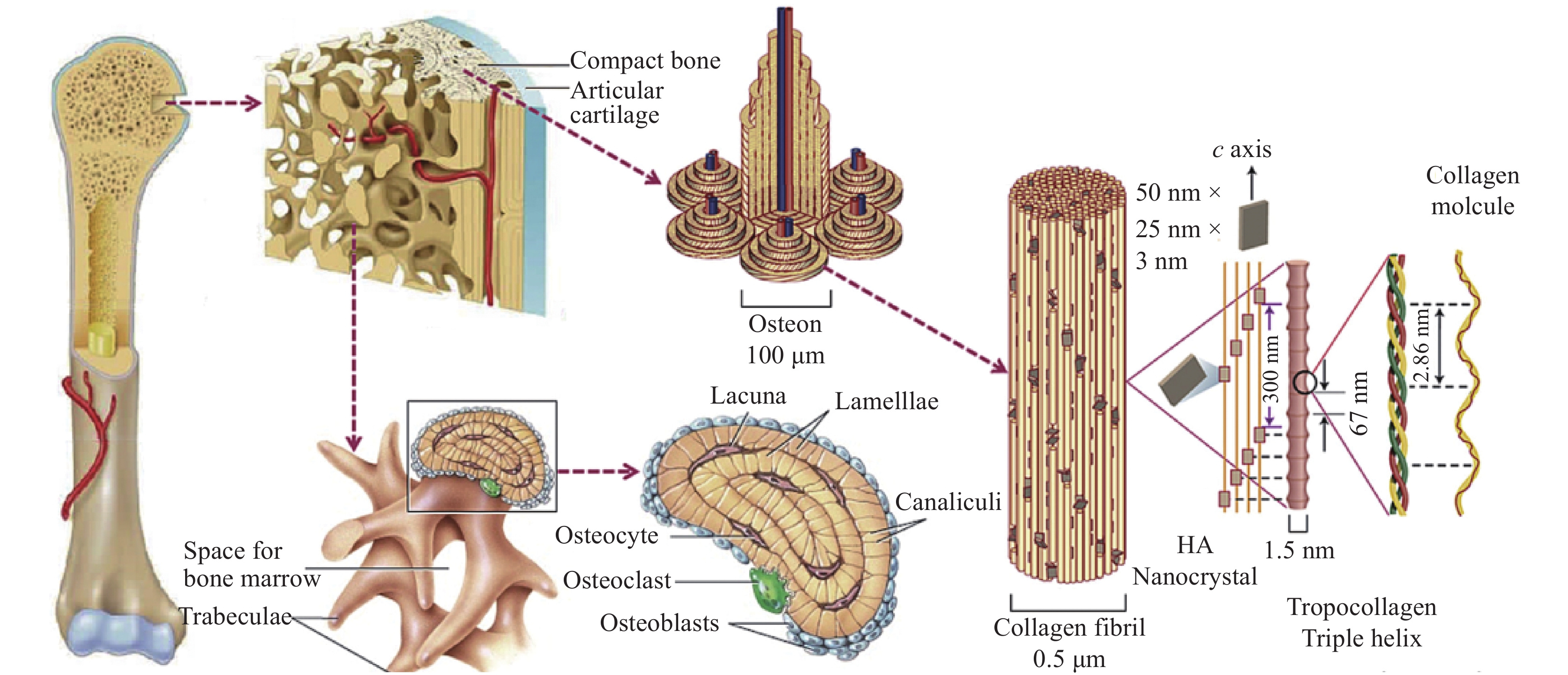
Bone tissue, being dynamic in nature, has the intrinsic capability to regenerate itself in response to damage or injury[1]. It undergoes continuous renewal and remodeling throughout its adult life. The organic matrix of osteoid is composed of collagen fibers and ground substances immersed in a semisolid gel. The bone cells that produce the organic matrix are known as osteoblasts, the bone cells that break down old bones are called osteoclasts, and mature osteoblasts that can no longer form new bones are called osteocytes[2] (See Figure 1). Nevertheless, the reconstruction of critical-sized bone defects remains a key challenge. In recent years, the global aging population has been in great demand for advanced bone substitutes. A case study by Global Burden of Disease has revealed that a total of 336.5 million prevalent cases and
74000 deaths are caused by musculoskeletal disorders such as arthritis and back pain[3]. Other bone-related defects are caused by trauma, abnormal growth, or tumors[4]. Another common skeletal disorder gaining attention is osteoporosis (OP), in which bones become so fragile and brittle that they result in fractures. In 2015, OP alone caused 1.3 million fractures in the US and 2.33 million fractures in China in 2010. It has been estimated that the numbers will exponentially rise in China, reaching up to 5.99 million by 2050[5]. Due to all these setbacks, the economy and the health of citizens worldwide are being compromised. To overcome this challenge, clinical treatments have been widely used, such as bone cement and bone grafting, which include autografts and allografts[6]. However, these have various limitations, including the short lifespan of grafts, donor site morbidity, infections, immune rejection, and limited donors[4]. In recent years, there has been a breakthrough in the regeneration of bone tissue through a tissue engineering technology called bone tissue engineering (BTE)[7]. Advances in BTE are reflected in the ability to achieve tissue regeneration through the construction of biodegradable porous scaffolds (See Figure 2). In Figure 2, collected stem cells, along with different nanoparticles and growth factors, are grown within the scaffold and then the scaffold loaded with cells is implanted in the defected area for regeneration[1]. Regeneration of bone tissues requires interplay between 3 components: scaffold material, growth factors that induce osteogenesis and angiogenesis, and cells undergoing osteogenic differentiation[8]. Three-dimensional (3D) bioprinting has shown significant potential in tissue engineering to regenerate impaired tissues due to its ability to create tissue-engineered scaffolds with aesthetic benefits in terms of structure over other traditional scaffold fabrication techniques[9]. Meskinfam et al.[10] produced a unique approach to characterize polymeric scaffolds for bone tissue engineering. The study characterized various pathways needed for BTE, concentrating on scaffold aspects such as morphology, architecture, structural properties, and surface chemistry.Chitosan is one of the popular natural polysaccharides that has been studied over time due to its tunable chemical and biological properties and is widely used in BTE applications. Chitosan stands out as an appealing bone scaffold material as it promotes osteoblast cell adhesion and proliferation, as well as the creation of a mineralized bone matrix[11]. Research findings show that there are some drawbacks related to the chitosan scaffold, such as water insolubility and weak mechanical strength; nevertheless, when combined with other polymers or ceramics, the material improves chitosan’s performance in bone healing applications[12]. On a positive note, chitosan tremendously resembles glycosaminoglycans (GAGs) found in the extracellular matrix (ECM), contributing to its non-toxic biocompatible nature. Thus, chitosan presents new avenues for bone treatments and stands out competitively as a versatile biopolymer in terms of its bioavailability, low production cost, and ability to elicit cell adherence, mineralization, and neovascularization[13].
1.1 Source and physio-chemical property of chitosan
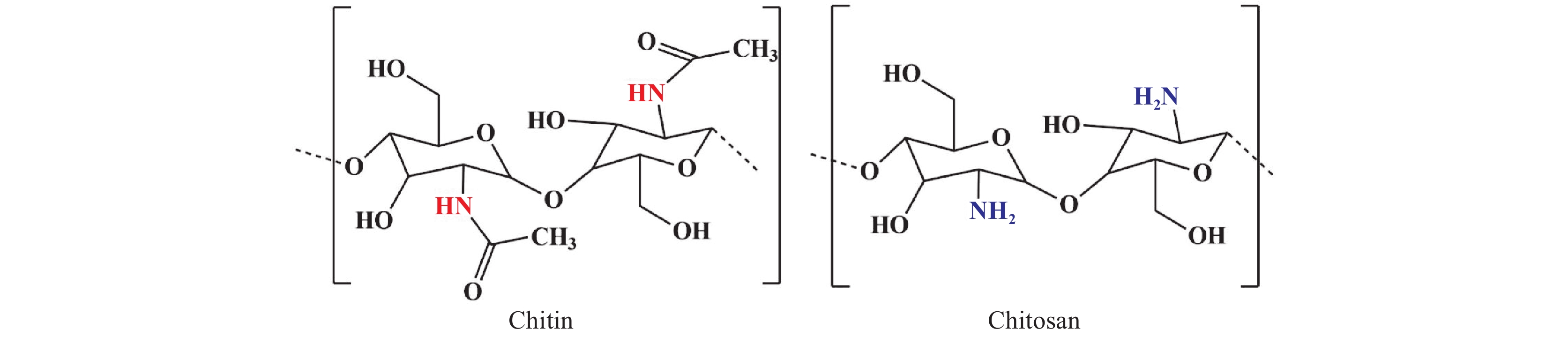
Crustaceans, mollusks, fungi, and other related organisms produce almost 100 billion tons of chitin each year. Chitin stands in the second position in terms of abundance after cellulose[14] and in terms of structure, it is a linear polysaccharide, made up of (1,4)-linked N-acetyl-D-glucosamine units. At the industrial level, chitin is extracted from its shell in 3 basic steps: deprotonation of chitin by adding an alkaline solution, demineralization by further treatment with an acidic medium, and finally discoloration[15]. From being available in nature in huge amounts to having exceptional features such as biodegradability, and biocompatibility, raw chitin severely lags behind when it comes to solubility. Henceforth, it makes us all shift towards chitosan, a derivative of chitin. Lengths of the polymer chain, deacetylation degree, and molecular weight are some major parameters required to determine the physical, chemical, and biological properties of the chitosan scaffold. In addition to these key parameters, there are other secondary criteria such as viscosity, crystallinity, coagulation, and solubility. The conversion of chitin into chitosan can be enzymatic or through a chemical process. The chemical process is widely preferred as the production expense is low and it is more suitable for mass production[16]. The reaction through which chitosan is obtained is chitin deacetylation where chitin is treated with a strong sodium hydroxide solution (40%–50%) at 100 ℃ or higher to remove some or all the acetyl groups, and the N-Acetyl-D-glucosamine and D-glucosamine repeating units linked by β-(1-4) glycoside bonds are randomly placed (See Figure 3). More precisely, when the concentration of deacetylation is up to 60%, chitosan becomes soluble in an acidic environment. The amino groups of chitosan protonate in the acidic media (pH < 4), become cationic in nature, thus enabling it to interact with a variety of molecules and making it the only cationic polysaccharide found in marine environments[17]. Moreover, chitosan resembles GAGs found in the ECM contributing to its non-toxic biocompatible nature. The physiochemical characteristics of chitosan like biodegradability, solubility, crystallinity are all greatly influenced by the degree of deacetylation[18]. The electrostatic interaction between the negatively charged bacterial cell envelope and positively charged chitosan is responsible for its antimicrobial activity too. Apart from its antimicrobial and antioxidant activity, chitosan is also used in wound healing, and has been approved by the Food and Drug Administration (FDA)[18]. Chitosan shows better bioactivity when its molecular mass is less than 20 kDa. Again viscosity is strongly related to the molecular weight of chitosan. In the aqueous acid medium, viscosity decreases as the solvent temperature increases. Chitosan also possesses excellent coagulating properties. In the presence of N2 or any metal ion, the amino group present in chitosan acts as an electron donor and is responsible for metal ion chelation. The majority of these characteristics are related to the chitosan’s backbone’s free protonable amino groups, which also contribute to its solubility in acidic solution. Additionally, chitosan biopolymer may be physically altered, opening up a number of shape possibilities. As a result, this polysaccharide is utilized in several industries, including tissue engineering, drug delivery systems, and also for the treatment of cancer.
1.2 Chitosan in bone tissue engineering
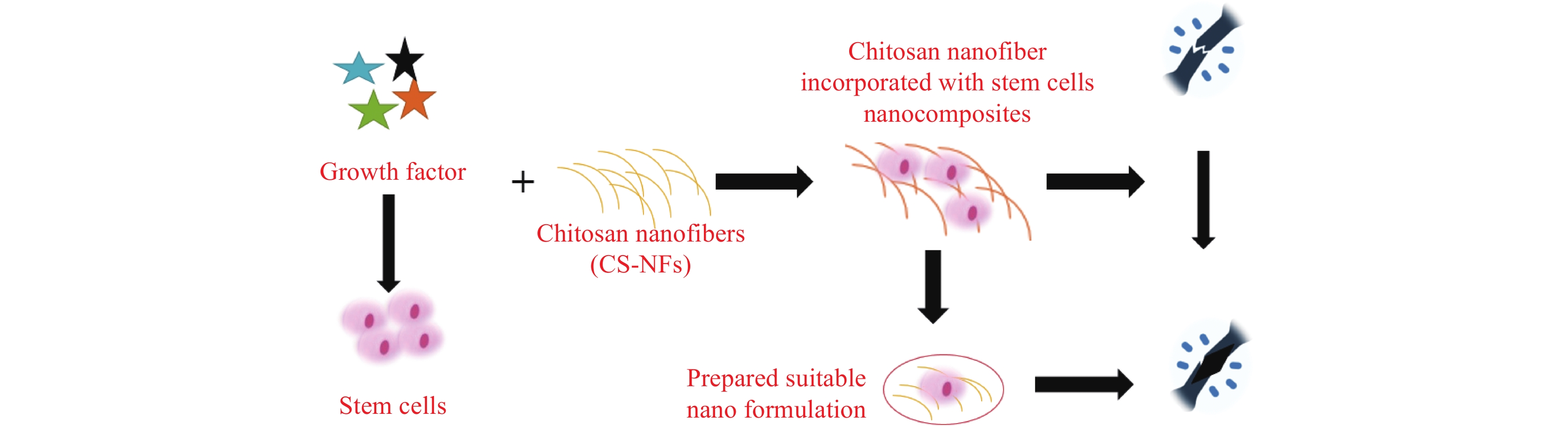
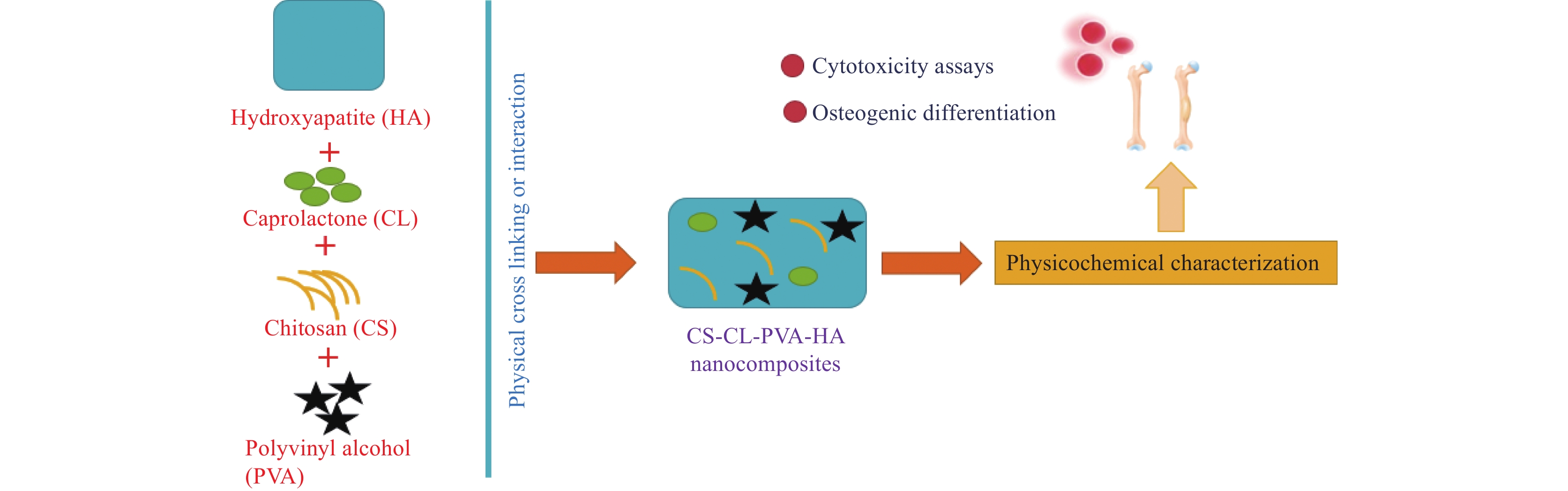
Chitosan has received a lot of interest in recent years for its usage as a graft material in BTE, either alone or in combination with other materials. Combining chitosan nanofibers with various growth factors infused within stem cells could potentially heal the damaged area (See Figure 4). Reyna-Urrutia et al.[19] has reported and provided remarkable remarks on BTE, stating that bone regeneration is an urgent therapeutic need for the treatment of bone abnormalities caused by a variety of reasons. Study indicates there is a clinical demand for chitosan based scaffolds that offer flexibility in scaffold production and facilitate effective progress in BTE[11]. Saravanan et al.[1] focuses on chitosan and its characteristics, as well as the role of chitosan and other polymeric and ceramic materials as scaffolds for bone tissue healing applications. Porosity, biocompatibility, water retention, protein adsorption, mechanical strength, biomineralization, and biodegradability are all desirable features in scaffolds for BTE applications. In reference [19], synthesized biocomposites CS-CL-PVA-HA, which are composed of chitosan (CS), hydroxyapatite (HA), caprolactone (CL), and polyvinyl alcohol (PVA), are evaluated against osteogenic activities. The CS-CL-PVA-HA scaffold demonstrated good cell survival, proliferation, and differentiation of dental pulp stem cells (DPSCs), indicating its potential for in vitro and in vivo bone defect research. The inclusion of HA improved its osteoconductive characteristics, allowing for more effective bone tissue regeneration (See Figure 5).
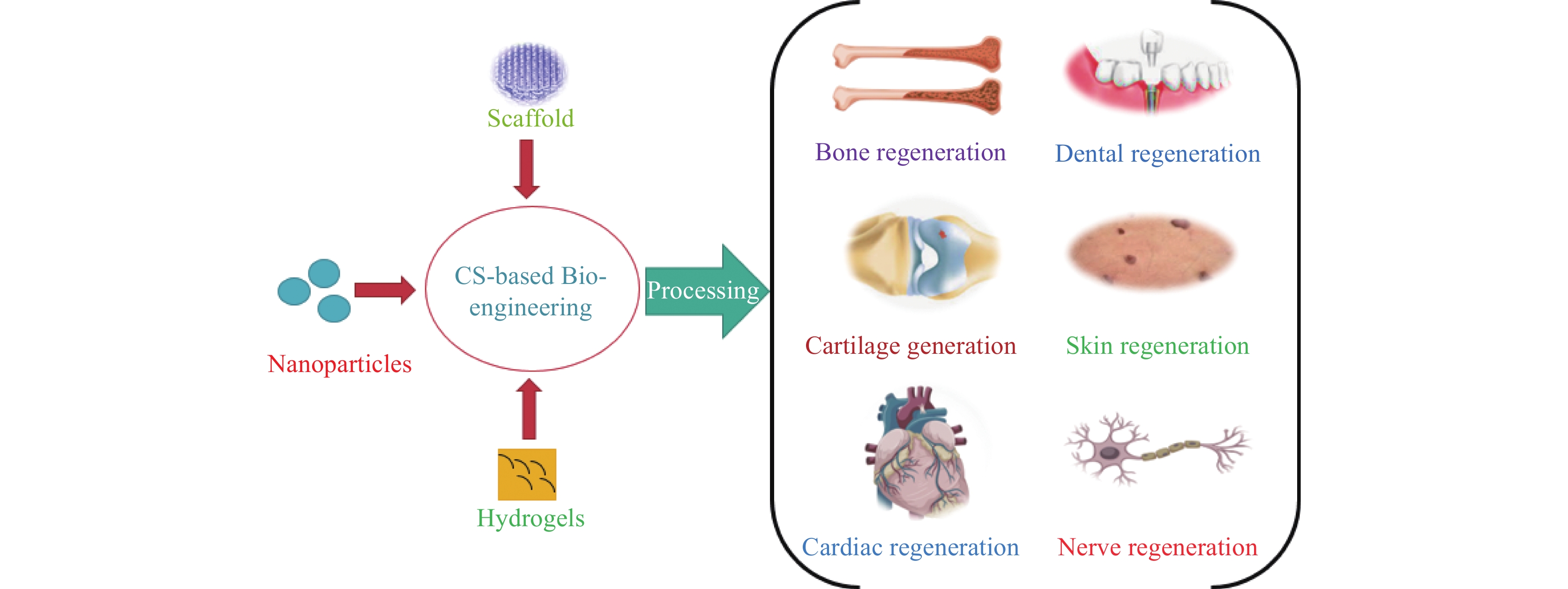
Kim et al.[20] describe chitosan biopolymer as possessing numerous properties that make it appealing for biological applications and enable it to be produced into a wide range of structures, including nanoparticles, scaffolds, hydrogels, and membranes, all of which can be tuned to achieve a desired result. It has been proven that composite chitosan-based biomaterials can be used in vivo regeneration and repair of numerous tissues and organs, including but not limited to bone, cartilage, dental, skin, nerve, cardiac, and other tissues. De novo tissue creation, resident stem cell differentiation, and extracellular matrix rebuilding were found in chitosan-based formulations treated preclinical models of various tissue lesions (See Figure 6). An in vitro study using a poly (lactic acid)/chitosan-based nanocomposite provided better mechanical stability and enhanced the production of calcium phosphate without any toxicity to the neighboring cells[21]. In another research[22], a chitosan/polyvinyl alcohol mesh in β tricalcium phosphate aerogel demonstrated effective bone renewal and proved that such a scaffold mesh could be a popular bone substitute candidate in biomedical engineering.
1.3 Chitosan biomaterial in cartilage regeneration
Injury to articular cartilages, which hold the synovial joints, results in premature arthritis and a poor quality of life if left untreated. The chondrocytes do not undergo mitosis and do not contain any blood vessels, thus the capacity of cartilage to regenerate is very low[23]. The available clinical treatment, such as chondrocyte implantation, have several drawbacks, such as the inability to completely fill the injured site, a lack of guarantee in forming hyaline cartilage, and a failure to integrate the repaired tissue with native tissue[24]. Therefore, alternative treatment using bone tissue engineering and regenerative medicines are of great interest. Based on past researches, materials infused with chitosan have shown positive outcomes, and due to its antimicrobial and biodegradable properties, it has proven to be one of the best alternative substances in cartilage engineering. The similar structure of the ECM of N-acetylglucosamine groups of chitosan and ECM of cartilage contributes to enhanced chondrogenesis[25]. Chitosan based hydrogels due to their ability to fill the injury site in any conceivable way and high water holding capacity showed great potential in clinical studies and composite hydrogels can be manipulated to have suitable mechanical and structural properties to enhance cell further proliferation and differentiation[26]. In an experiment, while delivering adipose derived mesenchymal stem cells (MSCs), the chitosan/dibenzaldehyde-terminated PEG hydrogel provided a suitable microenvironment for cell proliferation by facilitating an adequate supply of oxygen and nutrients. Further extending the experiment for better cytocompatibility and mechanical property, a hydrogel based on silanized hydroxypropylmethyl cellulose and silanized chitosan managed to demonstrate the secretory activity and viability of adipose-derived stem cells when the hydrogel was transplanted into the subcutis of nude mice[27]. When the same gel was injected into a canine, it showed osteochondral regeneration irrespective of the presence of adipose-derived stem cells. Thus, it depicts that the composition of hydrogel is capable of repairing osteochondral damages[27]. Biphasic chitosan scaffold in osteochondral engineering provides a scaffold that can compose numerous microenvironments at various levels. Utilizing the same feature, chitosan bilayered scaffolds were used, and buccal fat pad stem hypoxic cells were seeded into the same scaffold. The scaffold then expressed proteoglycans and reinforced COL2 thus proving the regeneration of hyaline cartilage[28]. Another study used a bilayered chitosan scaffold that included a hydroxyapatite porous bone layer and a thick cartilage layer. Furthermore, magnesium and copper ions were integrated into the bone and cartilage layers, respectively. Results demonstrated that Cu2+ ions promoted osteogenesis via enhanced expression of vascular endothelial growth factor-A (VEGF) and Mg2+ ions showed promoted cartilage regeneration. The bone layer of the scaffold provides mechanical strength by forming the subchondral bone and also upregulates the migration of new cartilage toward the synovial joint[29]. 3D tailored chitosan/poly(ε-caprolactone) hybrid scaffold embedded with synovial MSCs and a tetrahedral nucleic acid framework demonstrated in vivo regeneration. The scaffold upregulated the production of ECM components of cartilage and also showed the differentiation of synovial MSCs as the positively charged chitosan recruited the negatively charged nucleic acid[30]. Additionally, chitosan could be used as a multilayer scaffold for therapeutic release. In a study, a multilayer chitosan/polycaprolactone scaffold conjugated with ketogenic enhanced the chondrogenesis of MSCs[31].
1.4 Chitosan derivatives
Despite its numerous biomedical applications, whether alone or as a composite with other biomaterials and ceramic particles, the use of chitosan is still limited. A disadvantage is that chitosan’s solubility in aqueous solutions depends on pH and at neutral pH, the solubility of chitosan is limited. Moreover, the presence of glycosidic linkages makes chitosan easily degradable in the body due to the abundance of hydrolytic enzymes of lysozyme[32]. Therefore, the biocompatible nature of chitosan can be maximized when it is modified to a certain extent, which aids in improving its solubility and degradation properties. Table 1 comprises various scaffolds based on modified chitosan derivatives.
Table 1. Some common derivatives of chitosan and their featuresModified chitosan Composite In vitro studies Animal defect area and model Characteristics Methacrylate chitosan[33] Chitosan-β-glycerol phosphate salt/lithium phenyl-2,4,6- trimethylbenzoyl-phosphinate (LAP) photoinitiator Cell proliferation observed in all cell lines - The 3D hydrogel demonstrated improved cell adhesion and migration, confirming its cell compatibility and permeability Carboxymethyl chitosan[34] Polydopamine (PDA)/ hap Biocompatible and better cell adhesion of MC3T3-E1 mouse preosteoblastic cells Male New Zealand white rabbits, femoral bone defect The 3D scaffolds achieved a satisfactory level of biodegradability, balanced bone regeneration, and promoted the repair of cancellous bone defect N,O-carboxymethyl chitosan[35] Polyphosphate Induced Saos-2 cell mineralization 6-week-old male SD rats, calvarial defect Bone biomineralization and in vivo studies demonstrated significant regenerative inducing activity Quaternized chitosan[36] PLGA/Hap Human bone marrow-derived MSCs proliferated and differentiated towards the osteoblast lineage 6-week-old male SD rats and dorsum subcutaneous implantation model Exerted anti-bacterial effect and displayed strong tissue integration. Neovascularization upon in vivo implantation Quaternized chitosan[37] PLGA/Hap - Female SD rats: femoral shaft defect; female New Zealand white rabbits: condyle defect Demonstrated significant anti-infective effect and bone repairing ability in both the 2 bone defect models Phosphorylated chitosan/ disodium (1 → 4)-2- deoxy-2-sulfoaminoβ-dglucopyranuronan chitosan[38] PLGA/TCP - - Exerted remarkable mechanical properties and absorbability Phosphorylated chitosan/ disodium (1 → 4)-2- deoxy-2-sulfoaminoβ-dglucopyranuronan chitosan[39] PLGA/TCP - Mature female New Zealand white rabbits, critical-sized ulnar bone defect Possessed dual functions, including osteogenic and osteoclastic properties 1.4.1 Carboxymethyl chitosan
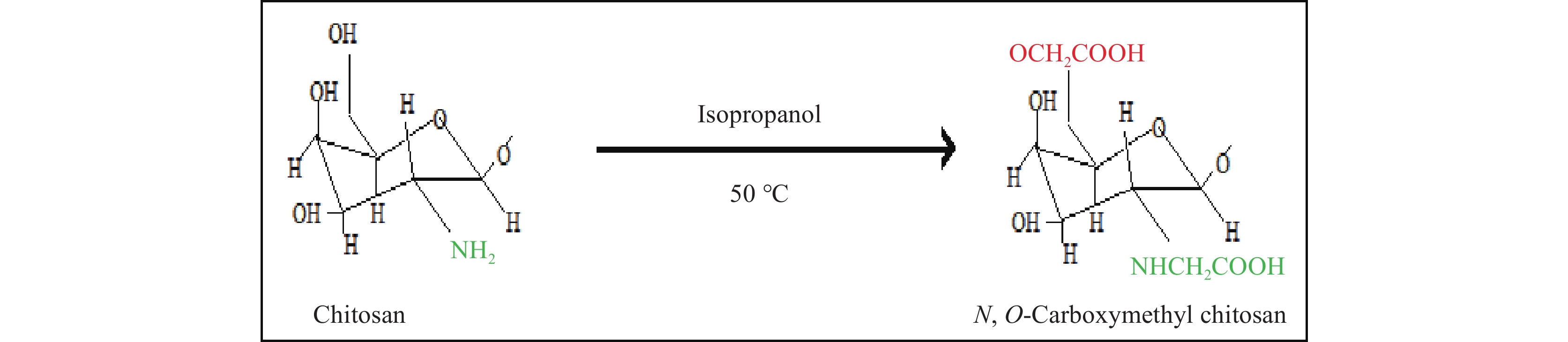
To improve chitosan solubility, one approach is to introduce functional groups, such as carboxyalkyl groups. Carboxymethyl chitosan (CMC) is obtained through a reaction at the C6 hydroxyl group or the NH2 group, resulting in products that are either N-CMC, O-CMC, or N-O-CMC. It is amphoteric and thus widely used in medical fields (See Figure 7). CMC polymer can show strong bioactivity when loaded with hydrophobic drugs[40]. The properties of CMC depend on the degree of substitution, which further depends on the molecular weight and the amount of carboxylate agent used. Molecular weight and degree of substitution are inversely proportional to each other[41]. When the carboxyl group of CMC is compared with other biopolymers, it greatly resembles alginate and agarose, both of which are highly compatible with CMC. An improvement in the tensile strength was observed when CMC was cross-linked with alginate and agarose in neural stem cell differentiation[42]. However, an in vitro study where ceria-infused CMC-coated hydroxyapatite nanocomposite delayed the release of nanoceria in nerve cells and failed to deliver anti-alzheimer’s agent[43]. CMC also increased the efficacy of compounds showing poor bioavailability and solubility[33]. The advantages of using CMC are as follows: improves adhesion and proliferation of osteoblasts, enhances mechanical strength, enhances osteoinductivity, and promotes new bone formation[44]. CMC has gained popularity in the pharmaceutical field due to its antibacterial, antiviral, antitumor, and lipid-lowering properties. Carboxymethyl chitosan/PVA electrospun scaffolds showed hMSC adherence and proliferation with no cytotoxicity[45]. Injectable gels of CMC-gelatin-nHAp enhanced the proliferation of osteoblasts and showed no inflammation at the site of implantation[46]. Carboxymethyl chitosan/gelatin/β-TCP composites produced through ultrasonic radiation showed better porosity in structure along with optimum mechanical strength. Micro-CT showed that the scaffold promoted biocompatibility and bone formation when implanted in the mandibular region of a canine model[32].
1.4.2 Acylated chitosan
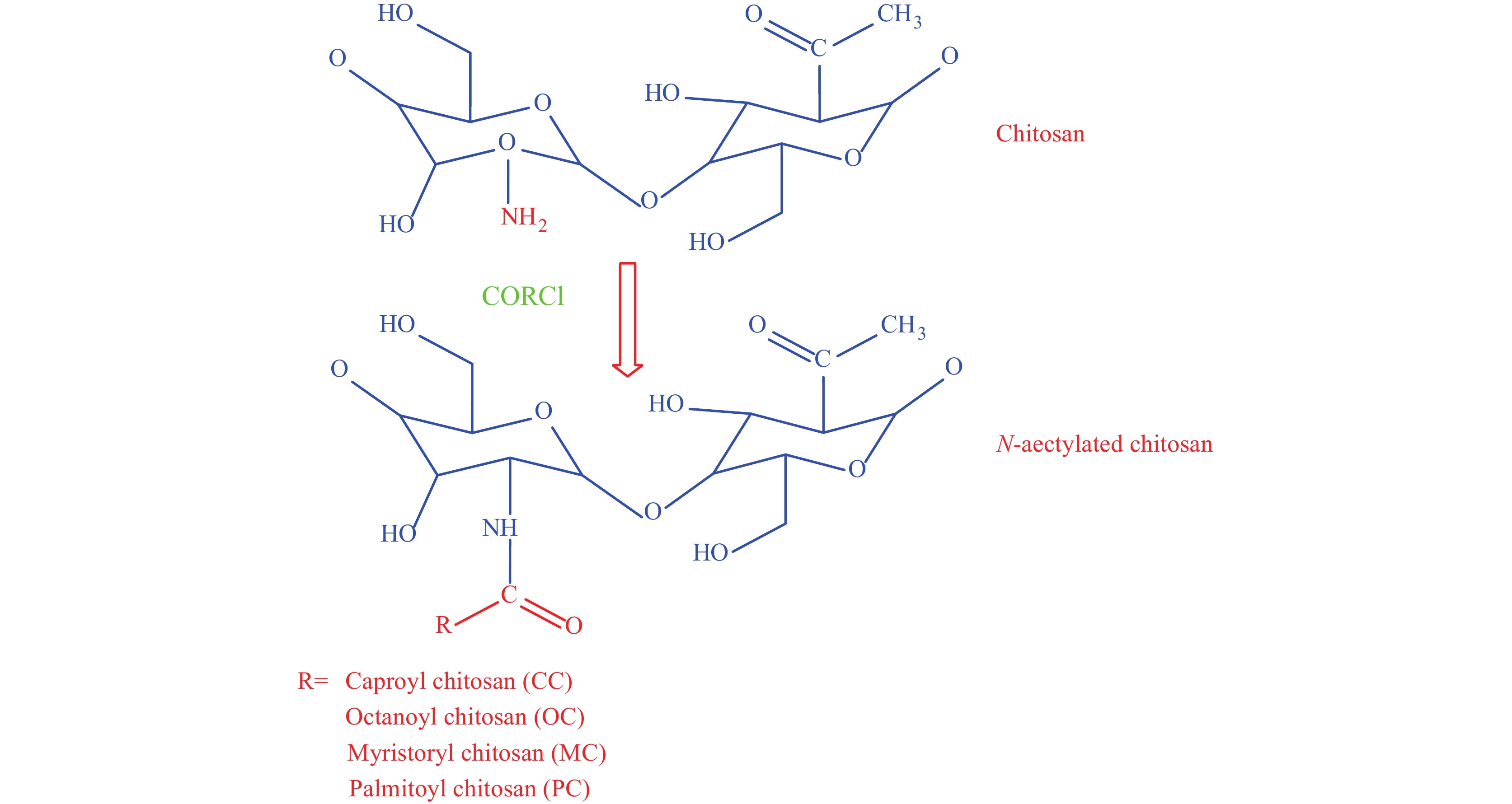
Another common modification is the acylation of chitosan. Here, multiple acyl groups (e.g., aliphatic and aromatic) are added to the chitosan chain, and the reaction is carried out with various organic acids (See Figure 8). The acylation disrupts inter- and intramolecular hydrogen bonding and increases solubility. There are 2 types of acylation: N-acylation and O-acylation. In N-acylation, the amide is formed by the reaction at the C2-NH2 group, and in O-acylation, the ester is formed by the reaction at the C6-OH group[47-48]. O-acylated chitosan is lipid-soluble and can dissolve in chloroform, and can be used for enhancing biomaterial stability[33]. N-acylated chitosan, on the other hand, shows anti-coagulant properties, biocompatibility, anti-inflammatory reactions, and is widely used as a carrier in biomedical applications.
1.4.3 Quaternary ammonium chitosan
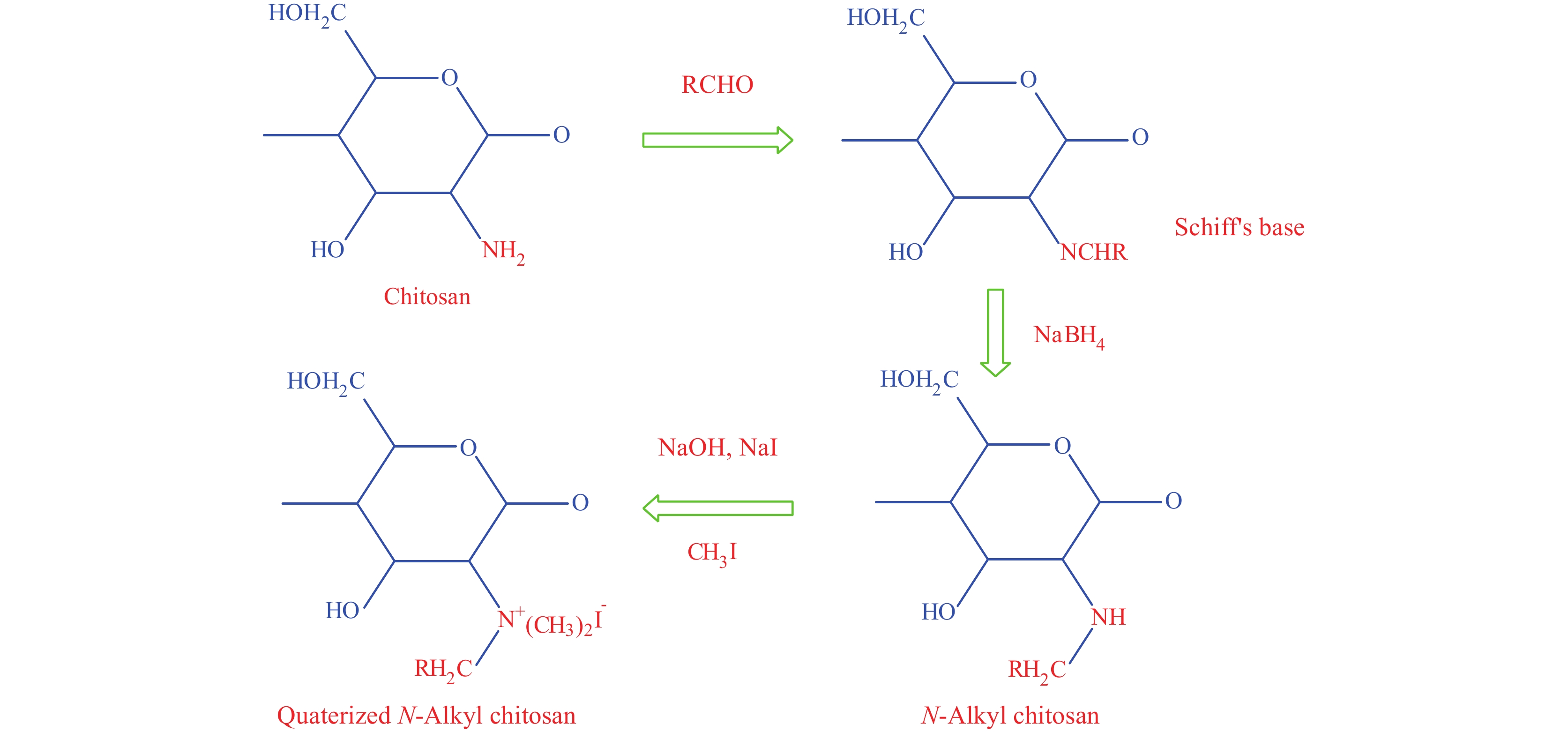
The quaternary ammonium chitosan derivative not only possess higher water-solubility property but also are stronger positively charged hydrophilic polymer than chitosan. Below a pH of 6.5, chitosan without any modifications is positively charged, however, above a pH of 6.5 free amino acids of chitosan deprotonate and become insoluble while quaternized chitosan retains its positive charge above pH 6.5, making it a more effective derivative in terms of antibacterial activity. The reaction for the formation of quaternary ammonium chitosan takes place at the C2-NH2 site, and an alkyl group is added in place of an amino group. N-trimethyl chitosan (TMC), the strongest muco-adhesive polymer is one of the products of the quaternized chitosan (See Figure 9). Quaternized chitosan has proven to have higher water solubility and stronger cationic property than chitosan[49]. A case study of TMC/heparin multilayer on cortical bone allograft showed similar behavior to that of periosteum, a fibrous covering present on the bone surface[50]. The anti-bacterial properties of TMC have also been studied and found to hinder the growth of both gram-positive and gram-negative bacteria[51]. Apart from non-toxicity and mucoadhesiveness, quaternary ammonium chitosan also shows higher antigen binding property and better cell permeability than chitosan alone, and are popularly used in medicine.
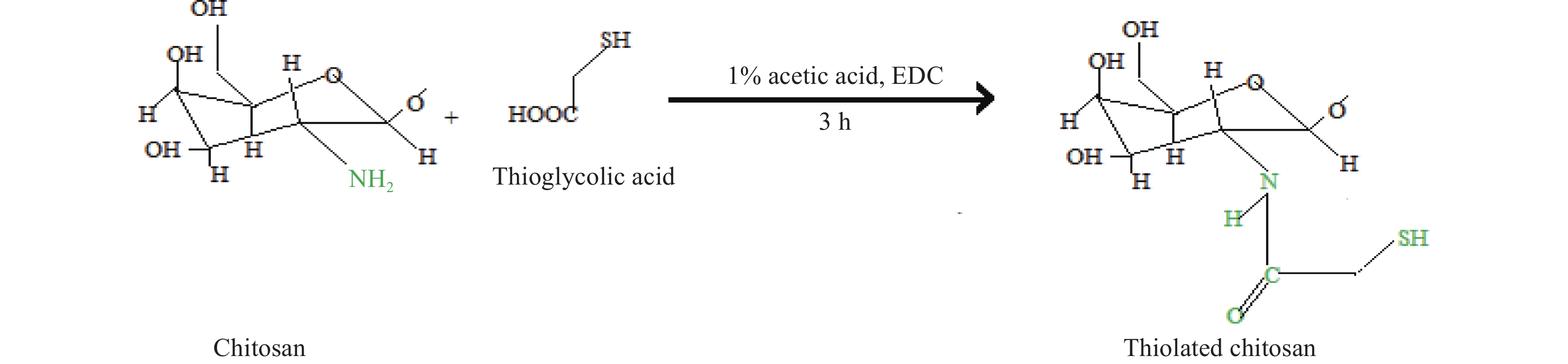
1.4.4 Thiolated chitosan
This is a significant modification of chitosan that not only enhances hydration but also provies in situ gelling capability. The reaction occurs between the thiol-containing coupling agent and the primary amino group of chitosan. The thiol group readily adheres to the cysteine present in mucus through the formation of a covalent bond, thus displaying better mucosal adhesion properties[52] (See Figure 10). Methacrylamide chitosan derivative can also be thiolated. In tissue engineering, thiolated chitosan is widely recognized for delivering growth factors such as VEGF and platelet-derived growth factors (PDGFs) for blood vessel regeneration. It also serves as a thermosensitive cell carrier for tissue regeneration. Other applications include the use of biodegradable bandages in wound treatment, anti-microbial coatings in polymer films, removal of As3+/As5+ from groundwater in wastewater treatment, and in cosmetics to prevent contact dermatitis[53].
2. Mechanical and biological properties of 3D bio-printed chitosan scaffold
2.1 Porosity
Proper pore dimensions are very crucial in scaffold fabrication as they support cell infiltration, cell adhesion, secretion of different ECM components, and tissue growth. Numerous studies highlight various optimal pore sizes; 20–100 μm is beneficial for cell infiltration, whereas >100 μm improves neovascularization. It has also been reported that improper pore size indirectly affects cell behavior, as small pores limit cell permeability and large pores limit ligand availability for cell-to-cell binding. Polymer concentration, printing technique, cross linkers, temperature, and the added number of nanoparticles (NPs) are basic parameters for pore designing in chitosan scaffolds. Genipin, ethyl-3[3-dimethylaminopropyl] carbodiimide hydrochloride (EDAC), and tripolyphosphate (TPP) are some common cross linkers that improve the morphology and interconnectivity of the pores as well as of the scaffold[54].
2.2 Mechanical property
The Young’s modulus and compressive strength of cortical bone are 15–20 GPa and 100–200 MPa, respectively, whereas cancellous bone possesses 0.1–2 GPa and 2–20 MPa, respectively[55]. Studies have shown a positive relationship between the stiffness of a scaffold and the behavior of cells nearby; it influences MSCs to differentiate into specific cell types. Hence, the scaffolds must possess an appropriate load-bearing capacity. Chitosan, on the other hand, has a moderate strength which limits its use. However, adding ceramics or other polymers to chitosan increases the tensile strength of the scaffold[56]. Namely, the addition of graphene (GR) within the chitosan scaffold enhances the strength of the scaffold. Another 3D bio-printed GR/chitosan/gelatin/hyaluronic acid scaffold influences MSCs to proliferate[57]. The incorporation of gelatin and tricalcium phosphate can also accelerate the strength of the scaffold by up to 70%[58]. Wu et al.[59] investigated the tensile strength and cell viability of a chitosan hydrogel observed under scanning electron microscope (SEM) and optical microscopy. For stress testing, the hydrogel underwent various processes such as neutralization, and non-neutralization before being tested using an Insight MTS 50 kN (electromechanical machine). For cell movement, L929 fibroblast cells were seeded on the hydrogel. The results showed that the post-neutralized scaffold had better tensile properties than the printed filament, due to the formation of complex inter- and intra-molecular hydrogen bonds. The filament, when tested using tweezers, was stretched for 1 min and then released, after which its complete recovery was observed. SEM imaging also displayed excellent fibroblast growth on the hydrogel, demonstrating chitosan as a suitable scaffold for cell multiplication.
2.3 Osteogenesis and vascularization activity
Chitosan scaffolds lacking vascular networks result in cellular apoptosis, and to overcome this, a novel strategy has been invented. The RGD peptide (Arginylglycylaspartic acid) embedded in CaP/chitosan porous scaffolds containing MSCs was implanted in the rat’s radial defect bone. The introduction promoted neovascularization along with osteoblastic proliferation[60]. 3D printed allyl chitosan also induces osteogenesis when hMSCs were cultured at an early stage[61]. The magnesium calcium silicate (Mg-CS/chitosan-coated) titanium alloy (Ti-6Al-4V) scaffold elicited surface hydrophilicity, which aided in the adherence, proliferation, and differentiation of hMSCs. A study revealed that a high percentage of new bone was formed in the Mg-CS/chitosan scaffold when evaluated histomorphometrically in femur defects of rabbits[62]. An attempt was made by fabricating CaP lattice-shaped 3D scaffolds using chitosan as a binding agent, and an extrusion-based technique was used. The results showed high expression of ALP, OCN, and COL1[63]. Likewise, another attempt using Mg/nHAp/chitosan/gel scaffold demonstrated not only high expression of osteoblast marker genes such as Runx2, OCN, and COL1, but also a significant increase in the attaching and differentiating capacity of osteogenic cells[64]. New bone formation in calvarial defects was also investigated when osteoblast-like cells were obtained from TCP/Col/chitosan hydrogels[65]. Chitosan/gelatin scaffold showed the formation of extracellular matrix in femur implantation in an orthotopic mouse model. Moreover, preosteoblastic cells and bone marrow stem cells successfully adhered and proliferated in the chitosan/gelatin scaffold and showed high expression of osteogenic markers such as ALP, and RUNX2. Membranes of chitosan/polycaprolactone with strontium and calcium phosphate nanoparticles resulted in angiogenesis by releasing vascular endothelial growth factor and also formed calcified nodules, thus proving the membrane’s capability for osteogenic and angiogenic differentiation. Chitosan/alginate multilayer films fabricated with interleukin-4 loaded on chitosan/alginate multilayer films provided a sustainable release of the cytokinin and enhanced angiogenic markers[66].
2.4 Swelling and biodegradation
In vivo, chitosan is mainly degraded by lysozyme found in every mammalian tissue (serum, saliva, other fluids), whereas in vitro, chitosan can be degraded via hydrolysis or oxidation. The biodegradation rate depends on the crystallinity, molecular mass, and length of the chain. Degraded chitosan releases monosaccharides, which either integrate with the metabolic pathway of GAGs or are excreted[67]. Chitosan degradation was demonstrated in rats where chitosan based hydrogels were injected into the back, and after 19 d, no traces of the gel were found, indicating complete resorption. Pure collagen and chitosan/collagen hydrogels’ swelling ratios and degradation showed that collagen alone had a maximal swelling rate, which disturbed the geometry of the scaffold. However, when chitosan was infused with collagen, it not only maintained the integrity by reducing the swelling rate but also inhibited the collagen from degradation. The degradation performance was tested for the scaffold and in the presence of collagenase type I, collagen alone completely degraded in 1 h at room temperature on a 100 rpm shaker. However, an equal proportion of collagen and chitosan showed only a 30% degradation rate and also decreased shrinkage of the overall 3D scaffolds. The tensile strength of the collagen/chitosan scaffold was also enhanced with an increase in the chitosan ratio. Thus the addition of chitosan in the collagen/chitosan scaffold improved the mechanical strength and biocompatibility of the scaffold[68]. The structural morphology of gelatin/alginate hydrogels was also improved by the addition of carboxymethyl chitosan (CMCS). Moreover, the degradation rate of the hydrogel was also slowed down by CMCS[69].
2.5 Immunoadjuvant property
In response to tumors, chitosan biologically exhibits the ability to activate macrophages to produce nitric oxide (NO) and interleukin 1 (IL-1). It also acquires the ability to respond to chemical stimuli like neutrophils[70]. 3D chitosan sponges have shown that a higher degree of deacetylation leads to increased levels of osteopontin (OPN), IL-6, vascular endothelial growth factor-A (VEGF), and alkaline phosphatase (AP), however a lower degree of deacetylation results in increased secretion of sclerostin (SOST) and osteoprotegerin[71].
2.6 Antioxidant and antibacterial activity
Structurally, chitosan is a cationic polysaccharide consisting of amino groups (NH2) and becomes soluble under a pH level of 6.5. Its positive charge and functionally active amino groups are responsible for the antimicrobial activity of chitosan, making it interesting for biomedical applications. Chitosan has been experimentally shown to inhibit the proliferation of various bacteria, fungi, and yeasts through different mechanisms, some of which are not fully clarified. One of the simplest mechanisms of action involves electrostatic interactions between the NH3+ sites of chitosan, which is positively charged, and the membranes of microbial cells (negatively charged). The interaction disrupts the permeability of the microbial cell, causing the release of intracellular material. Chitosan also sometimes interacts with peptidoglycan and alters the osmotic gradient of the membrane wall.
It has been revealed that a 0.02% chitosan solution in vitro has positive antioxidant effects, such as reducing lipid peroxidation and enhancing antioxidant enzymes like glutathione peroxidase (GSH-PX), superoxide dismutase (SOD), and catalase (CAT). The higher the degree of deacetylation, the better the antimicrobial effect[72]. The incorporation of CMCS in gelatin/alginate hydrogel also aids antibacterial activity and provides better mechanical stability to the scaffold[69]. Photocured chitosan-Ph hydrogel (phenolic hydroxyl moiety) demonstrated suppressed bacterial growth when Escherichia coli (Gram-negative bacteria) and Staphylococcus aureus (Gram-positive bacteria) were tested for antimicrobial activity.
For bone reconstruction, chitosan scaffolds can be used solely with bioactive molecules, or they can be combined with other materials such as ceramics, synthetic, or any other natural polymers. Chitosan blends perfectly with other polymers, and with proper adjustments, chitosan scaffolds show positive effects in medical fields as shown in Table 2.
Table 2. Chitosan-based 3D scaffolds and their characteristics for bone tissue reconstructionPolymers Preparation methods Animal model and defect areas Comments Chitosan/silk fibroin/cellulose nanoparticle[73] BP (bioprinting) Calvarial defect in a rat model The osteo-immunomodulatory potential of the printed 3D scaffold was analyzed in hBMSCs and revealed improved bone formation. Hydrogel also showed better recovery strength Chitosan-vanillin-bioglass[74] BP Inbred C57BL/6NHsd female mice (6 weeks, ENVIGO) The mechanical property and bioactivity were improved in the scaffold, and in vivo studies also revealed better anti-bacterial property and osteoconductivity in vivo, data showed scaffold-supported BMP2-induced ectopic ossicle formation in mice Gelatin/chitosan/polyvinyl alcohol/nano-hydroxyapatite (GCPH)[75] - 4-week-old sprague-
dawley ratsThe GCPH scaffolds exhibited improved properties in terms of strength, adherence, osteogenic potential, porosity, proliferation, and biodegradation Regenerated cellulose nanofiber(Rcl)-chitosan[76] - - Hydrogel demonstrated better biomineralization and enhanced viability of pre-osteoblast cell (MC3T3-E1) was also enhanced Microchannel chitosan[77] Leaching method and freeze-drying 6 male 12-week-old sprague dawley (SD) rats Microchannel architecture scaffolds showed better cell survival, better proliferation, and migration in vitro, and encouraged tissue ingrowth in vivo Chitosan/gel/hap[78] fused deposition modelling (FDM) - scaffolds exhibited good porosity and oxygen plasma treated scaffold exhibited enhanced roughness, wettability, and higher MC3T3-E1 cell proliferation PLA/β-TCP/CP-amoxicillin[79] BP - Effective antimicrobial property, highest biocompatibility, structural stability, cell viability, proper porosity, and compressed strength was exhibited Mg-CLS/chitosan coated Ti-6Al-4V[62] Selective laser melting New Zealand rabbits- femoral bone defects Surface-modified scaffold showed better cellular functions such as cell adhesion, proliferation, and differentiation, and promoted osteogenesis and mineralization PHBV/CaSH/chitosan[80] FDM Adult male sprague dawley (SD) rats - rat ectopic osteogenesis model Increased rBMSC osteogenesis by upregulating the expression of osteogenic genes such as RUNX2, COL1, OCN, OPN, and BMP2 PLGA/nHAp/chitosan with rhBMP2[81] low-temperature deposition manufacturing (LTDM) 13-week-old New Zealand white rabbits- mandibular
bone defectSustained release of rhBMP2, biocompatibility in vitro, and 45.5% new bone formation in the defect site in vivo PCL/chitosan[82] FDM 7-week-old male nude mice subcutaneous implantation Scaffold possessed high compressive strength and a favorable bone matrix formation and osteogenesis BG/chitosan (NELL1) carrying BMSCs[83] Fiber deposition Adult female rhesus monkeys alveolar bone defect Model X-ray and micro-CT observations showed that the new bone was like the native bone in terms of mass, density, hardness, and shape GR/gel/chitosan/ TCP[84] BP - Showed better antimicrobial properties by the scaffold and alkaline phosphatase activity was also observed. Moreover, the printed material exhibited good osteogenic proliferation Hap/chitosan/genipin[85] Direct ink writing - Cyto-friendly environment, therefore, showing enhanced adhesion of human osteoblastic cells (MG63) to the scaffolds Chitosan/PVA/HAp with BMP2[86] BP - The elastic modulus of the chitosan scaffold closely matches that of natural bone, good biocompatibility, improved MSC attachment, and proliferation Chitosan/bioglass 46S6 with infused gentamicin sulfate[87] Freeze drying - Scaffold exhibited better release kinetics of gentamicin sulfate in vitro Chitosan-bioglass and carbon nanotube[88] Novel hot press and salt leaching process - Compressive strength nearly matched with the cancellous bone, better biodegradability, and promoted attachment and proliferation of MG-63 cells Chitosan/bioactive glass/PLGA nanoparticles[89] Freeze drying - Mechanical strength was increased, and the swelling behavior of the scaffold was lowered Chitosan-fucoidan-tri-calcium phosphate[90] Freeze drying - Improved compression strength was improved and enhanced apatite deposition was observed Alkali treated chitosan[91] 3D freeze drying - Up to 97%, the scaffold showed inhibition against bacterial growth as well as the ability to produce hydroxyapatite in vitro Ionic chitosan hydrogels (NaOH)[92] Extrusion-based - Simple preparation, self-assembly in an aqueous medium, low cytotoxicity 3. Commercially available and ongoing clinical trials of chitosan products
As previously mentioned, chitosan, which is approved by the FDA, is extensively utilized in the biomedical field, not just for bone regeneration but also for its potential in drug delivery, weight loss medication, hypocholesterolemic agents, hemodialysis, and absorbable sutures.
BST-Gel®, manufactured by Piramal Healthcare Canada Ltd., is a chitosan-based self-forming hybrid composite. It is an injectable mineral polymer that comprises 2 components: a liquid component (water-based, thermally sensitive) containing chitosan and an organic mono-phosphate source, and a mineral component containing Ca2+, F, SrCO3. These components are mixed to form an injectable paste. In situ, after injection, the slurry is converted into a gel-like substance. The product is utilized in bone filling, cartilage repair, and restoring intervertebral discs[93]. Other products are summarized in Table 3.
Table 3. List of chitosan-based medications available and their applications[94]Product (year of launch) Manufacturer Description Application BST-Gel® (2015) Piramal Healthcare Canada Ltd. Chitosan scaffold for cartilage repair Chronic wound healing, intervertebral disc restoration, bone filling, and cartilage repair HemconChitoGauze® (2010) XR PRO Tricol Biomedical Inc Hemostatic dressing Wound dressing CELOX RAPID (2011) - Celox rapid hemostatic gauze works with just 60 s of compression Quickest acting gauze, 60 s compression stops the severe arterial bleeding SILVAPRO (-) - An antibacterial burn dressing of size 5″×9″ is a combination of chitosan and ionic silver In a single solution it gives dual cooling as well as antibacterial protection Chitosan fibers (2021) ChiPro GmbH Chitosan fiber Textile or medical M-Chitosan (-) M-Chitosan The fabric in the inner layer remains 99.9 % antibacterial even after 100 washes Masks are reusable and washable Talymed® (2015) Marine polymer technologies, Inc. Sterile wound matrix Wound dressing material Reaxon® (2018) Medovent Nerve guide Nerve regeneration, hemostatic CELOX VASCULAR (2010) - 2×2-inch sized hemostatic gauze patch Local management of surface bleeding from vascular access sites, percutaneous catheters, or tubes There are numerous clinical trials ongoing with chitosan-containing bioactive products for the treatment of various diseases, wound healing, and many more, as summarized in Table 4 (https://www. clinicaltrials.gov/) (as accessed on Feb 11, 2023).
Table 4. Ongoing clinical studies of various chitosan-based drugs in medical fieldNTC number Title Status Disease condition Intervention Outcome Phase Location NCT02668055 Tb4 collagen and chitosan porous sponge scaffolds skin substitute treatment is difficult to heal wounds Completed Wounds TB4 Wound regression 1 China NCT05214807 Long-term safety and performance of Kiomedine CM-chitosan supplementation in advanced symptomatic osteoarthritis Recruiting Knee osteoarthritis KiOmedine® CM-chitosan Synvisc-One® Knee pain improvement 4 Belgium NCT02081885 Tricalcium phosphate and chitosan as bone regenerator versus autologous graft in surgery for mandibular fracture - Mandibular fractures Chitosan graft Bone density 3 Mexico NCT04365270 Antibacterial effect and clinical performance of chitosan modified glass ionomer Completed Dental caries Chitosan glass ionomer Antibacterial activity 3 Egypt NCT01895933 Efficacy and safety of the investigational device, SurgiShield anti-adhesion barrier gel Completed Wound healing 5 mL surgishield Adhesion rate 1 Korea NCT05333211 Ortho-R® for rotator cuff repair compared with standard of care rotator cuff repair without OrthoR® Recruiting Rotator cuff tears Ortho-R/PRP - 1 & 2 United States 4. Conclusion and future outlook
This review consolidates and provides insight into the past and current trends being followed in BTE using chitosan-based scaffolds. Chitosan exhibits a promising role as a biomaterial for BTE. However, certain limitations must be addressed. First, the chitosan is not thermoplastic and can easily decompose at high temperatures however blending it with thermoplastic polymers like polyvinyl alcohol can improve the thermal capacity of the film. Secondly, chitosan alone shows poor cell adhesion and requires simple alterations or modifications to enhance the adhering capability. Furthermore, solubility and mechanical properties also limit its application, which is why it is often blended with other polymers. Chitosan as a compound is insoluble in water as well as in most organic solvents, which limits both its usage and scope in biomedical fields. However, chitosan, containing active functional groups, can be modified through chemical reactions to obtain various derivatives. Chitosan derivatives are popular because they show a greater extent of solubility, pH-sensitive targeting, and increased delivery systems, and are in higher demand. The muco-adhesiveness, biodegradability, antimicrobial properties, and many more features make chitosan a more attractive biopolymer than other materials. Additionally, derivatives of chitosan are in greater demand, and their properties are further improved via chemical modifications. By combining with other materials and embedding bioactive compounds, 3D bioprinting of chitosan biopolymer has reached a new level, and it is now considered a biopolymer of the future. In the coming years, we believe that cutting-edge technologies will lead to extensive new research and variations using chitosan which will bring greater prospects in the biomedical field. Moreover, chitosan hydrogels are not only limited to bone regeneration; they can also be constructed and applied in other fields of interest such as vascular regeneration, periodontal regeneration, cartilage, drug delivery, wound healing, and many more. Altogether, this review helps to broaden our understanding of the development of chitosan hydrogels for future clinical applications.
5. Challenges
Firstly, to understand the maximum potential of bioinks, more knowledge in research and development is required. Bioinks are still in their infancy, as there is not a single bioink material that fulfills all the biological properties required for bioprinting. Secondly, although the 3D bioprinting method carries high potential in customizing desired products, it still lacks the ability to control pore size, its distribution, printing scaffolds, and cells, even with complicated shapes, and to biomimic the whole structure and organ properly. Thirdly, there is a scarcity of animal models. Currently, most experimental models are healthy, and there are few diseased animals available for study. Fourthly, the transportation and storage of hydrogels are challenging because they are fragile and tend to get damaged easily, which creates problems in experimental procedures. Lastly, the rapid disintegration and poor mechanical properties of chitosan hydrogel hinders their other beneficial properties. If the above-mentioned concerns are properly addressed, then the clinical research of chitosan hydrogels in bone regeneration can be achieved with precision.
-
Table 1 Some common derivatives of chitosan and their features
Modified chitosan Composite In vitro studies Animal defect area and model Characteristics Methacrylate chitosan[33] Chitosan-β-glycerol phosphate salt/lithium phenyl-2,4,6- trimethylbenzoyl-phosphinate (LAP) photoinitiator Cell proliferation observed in all cell lines - The 3D hydrogel demonstrated improved cell adhesion and migration, confirming its cell compatibility and permeability Carboxymethyl chitosan[34] Polydopamine (PDA)/ hap Biocompatible and better cell adhesion of MC3T3-E1 mouse preosteoblastic cells Male New Zealand white rabbits, femoral bone defect The 3D scaffolds achieved a satisfactory level of biodegradability, balanced bone regeneration, and promoted the repair of cancellous bone defect N,O-carboxymethyl chitosan[35] Polyphosphate Induced Saos-2 cell mineralization 6-week-old male SD rats, calvarial defect Bone biomineralization and in vivo studies demonstrated significant regenerative inducing activity Quaternized chitosan[36] PLGA/Hap Human bone marrow-derived MSCs proliferated and differentiated towards the osteoblast lineage 6-week-old male SD rats and dorsum subcutaneous implantation model Exerted anti-bacterial effect and displayed strong tissue integration. Neovascularization upon in vivo implantation Quaternized chitosan[37] PLGA/Hap - Female SD rats: femoral shaft defect; female New Zealand white rabbits: condyle defect Demonstrated significant anti-infective effect and bone repairing ability in both the 2 bone defect models Phosphorylated chitosan/ disodium (1 → 4)-2- deoxy-2-sulfoaminoβ-dglucopyranuronan chitosan[38] PLGA/TCP - - Exerted remarkable mechanical properties and absorbability Phosphorylated chitosan/ disodium (1 → 4)-2- deoxy-2-sulfoaminoβ-dglucopyranuronan chitosan[39] PLGA/TCP - Mature female New Zealand white rabbits, critical-sized ulnar bone defect Possessed dual functions, including osteogenic and osteoclastic properties Table 2 Chitosan-based 3D scaffolds and their characteristics for bone tissue reconstruction
Polymers Preparation methods Animal model and defect areas Comments Chitosan/silk fibroin/cellulose nanoparticle[73] BP (bioprinting) Calvarial defect in a rat model The osteo-immunomodulatory potential of the printed 3D scaffold was analyzed in hBMSCs and revealed improved bone formation. Hydrogel also showed better recovery strength Chitosan-vanillin-bioglass[74] BP Inbred C57BL/6NHsd female mice (6 weeks, ENVIGO) The mechanical property and bioactivity were improved in the scaffold, and in vivo studies also revealed better anti-bacterial property and osteoconductivity in vivo, data showed scaffold-supported BMP2-induced ectopic ossicle formation in mice Gelatin/chitosan/polyvinyl alcohol/nano-hydroxyapatite (GCPH)[75] - 4-week-old sprague-
dawley ratsThe GCPH scaffolds exhibited improved properties in terms of strength, adherence, osteogenic potential, porosity, proliferation, and biodegradation Regenerated cellulose nanofiber(Rcl)-chitosan[76] - - Hydrogel demonstrated better biomineralization and enhanced viability of pre-osteoblast cell (MC3T3-E1) was also enhanced Microchannel chitosan[77] Leaching method and freeze-drying 6 male 12-week-old sprague dawley (SD) rats Microchannel architecture scaffolds showed better cell survival, better proliferation, and migration in vitro, and encouraged tissue ingrowth in vivo Chitosan/gel/hap[78] fused deposition modelling (FDM) - scaffolds exhibited good porosity and oxygen plasma treated scaffold exhibited enhanced roughness, wettability, and higher MC3T3-E1 cell proliferation PLA/β-TCP/CP-amoxicillin[79] BP - Effective antimicrobial property, highest biocompatibility, structural stability, cell viability, proper porosity, and compressed strength was exhibited Mg-CLS/chitosan coated Ti-6Al-4V[62] Selective laser melting New Zealand rabbits- femoral bone defects Surface-modified scaffold showed better cellular functions such as cell adhesion, proliferation, and differentiation, and promoted osteogenesis and mineralization PHBV/CaSH/chitosan[80] FDM Adult male sprague dawley (SD) rats - rat ectopic osteogenesis model Increased rBMSC osteogenesis by upregulating the expression of osteogenic genes such as RUNX2, COL1, OCN, OPN, and BMP2 PLGA/nHAp/chitosan with rhBMP2[81] low-temperature deposition manufacturing (LTDM) 13-week-old New Zealand white rabbits- mandibular
bone defectSustained release of rhBMP2, biocompatibility in vitro, and 45.5% new bone formation in the defect site in vivo PCL/chitosan[82] FDM 7-week-old male nude mice subcutaneous implantation Scaffold possessed high compressive strength and a favorable bone matrix formation and osteogenesis BG/chitosan (NELL1) carrying BMSCs[83] Fiber deposition Adult female rhesus monkeys alveolar bone defect Model X-ray and micro-CT observations showed that the new bone was like the native bone in terms of mass, density, hardness, and shape GR/gel/chitosan/ TCP[84] BP - Showed better antimicrobial properties by the scaffold and alkaline phosphatase activity was also observed. Moreover, the printed material exhibited good osteogenic proliferation Hap/chitosan/genipin[85] Direct ink writing - Cyto-friendly environment, therefore, showing enhanced adhesion of human osteoblastic cells (MG63) to the scaffolds Chitosan/PVA/HAp with BMP2[86] BP - The elastic modulus of the chitosan scaffold closely matches that of natural bone, good biocompatibility, improved MSC attachment, and proliferation Chitosan/bioglass 46S6 with infused gentamicin sulfate[87] Freeze drying - Scaffold exhibited better release kinetics of gentamicin sulfate in vitro Chitosan-bioglass and carbon nanotube[88] Novel hot press and salt leaching process - Compressive strength nearly matched with the cancellous bone, better biodegradability, and promoted attachment and proliferation of MG-63 cells Chitosan/bioactive glass/PLGA nanoparticles[89] Freeze drying - Mechanical strength was increased, and the swelling behavior of the scaffold was lowered Chitosan-fucoidan-tri-calcium phosphate[90] Freeze drying - Improved compression strength was improved and enhanced apatite deposition was observed Alkali treated chitosan[91] 3D freeze drying - Up to 97%, the scaffold showed inhibition against bacterial growth as well as the ability to produce hydroxyapatite in vitro Ionic chitosan hydrogels (NaOH)[92] Extrusion-based - Simple preparation, self-assembly in an aqueous medium, low cytotoxicity Table 3 List of chitosan-based medications available and their applications[94]
Product (year of launch) Manufacturer Description Application BST-Gel® (2015) Piramal Healthcare Canada Ltd. Chitosan scaffold for cartilage repair Chronic wound healing, intervertebral disc restoration, bone filling, and cartilage repair HemconChitoGauze® (2010) XR PRO Tricol Biomedical Inc Hemostatic dressing Wound dressing CELOX RAPID (2011) - Celox rapid hemostatic gauze works with just 60 s of compression Quickest acting gauze, 60 s compression stops the severe arterial bleeding SILVAPRO (-) - An antibacterial burn dressing of size 5″×9″ is a combination of chitosan and ionic silver In a single solution it gives dual cooling as well as antibacterial protection Chitosan fibers (2021) ChiPro GmbH Chitosan fiber Textile or medical M-Chitosan (-) M-Chitosan The fabric in the inner layer remains 99.9 % antibacterial even after 100 washes Masks are reusable and washable Talymed® (2015) Marine polymer technologies, Inc. Sterile wound matrix Wound dressing material Reaxon® (2018) Medovent Nerve guide Nerve regeneration, hemostatic CELOX VASCULAR (2010) - 2×2-inch sized hemostatic gauze patch Local management of surface bleeding from vascular access sites, percutaneous catheters, or tubes Table 4 Ongoing clinical studies of various chitosan-based drugs in medical field
NTC number Title Status Disease condition Intervention Outcome Phase Location NCT02668055 Tb4 collagen and chitosan porous sponge scaffolds skin substitute treatment is difficult to heal wounds Completed Wounds TB4 Wound regression 1 China NCT05214807 Long-term safety and performance of Kiomedine CM-chitosan supplementation in advanced symptomatic osteoarthritis Recruiting Knee osteoarthritis KiOmedine® CM-chitosan Synvisc-One® Knee pain improvement 4 Belgium NCT02081885 Tricalcium phosphate and chitosan as bone regenerator versus autologous graft in surgery for mandibular fracture - Mandibular fractures Chitosan graft Bone density 3 Mexico NCT04365270 Antibacterial effect and clinical performance of chitosan modified glass ionomer Completed Dental caries Chitosan glass ionomer Antibacterial activity 3 Egypt NCT01895933 Efficacy and safety of the investigational device, SurgiShield anti-adhesion barrier gel Completed Wound healing 5 mL surgishield Adhesion rate 1 Korea NCT05333211 Ortho-R® for rotator cuff repair compared with standard of care rotator cuff repair without OrthoR® Recruiting Rotator cuff tears Ortho-R/PRP - 1 & 2 United States -
[1] Saravanan S, Leena RS, Selvamurugan N. Chitosan based biocomposite scaffolds for bone tissue engineering [J]. International Journal of Biological Macromolecules, 2016, 93: 1354-1365. DOI: 10.1016/j.ijbiomac.2016.01.112
[2] Meskinfam M. Polymer scaffolds for bone regeneration [M]// Characterization of Polymeric Biomaterials. UK: Woodhead Publishing, 2017: 441-475.
[3] Safiri S, Kolahi AA, Cross M, et al. Global, regional, and national burden of other musculoskeletal disorders 1990–2017: results from the global burden of disease study 2017 [J]. Rheumatology, 2021, 60(2): 855-865. DOI: 10.1093/rheumatology/keaa315
[4] Udduttula A, Teng B, Chandrashekar BN, et al. Novel Sr5(PO4)2SiO4-graphene nanocomposites for applications in bone regeneration in vitro [J]. Applied Surface Science, 2020, 507: 145176. DOI: 10.1016/j.apsusc.2019.145176
[5] Teng B, Yu XF, Li J, et al. Cervical vertebrae for early bone loss evaluation in osteoporosis mouse models [J]. Quantitative Imaging in Medicine and Surgery, 2023, 13(4): 2466. DOI: 10.21037/qims-22-717
[6] Murizan NIS, Mustafa NS, Ngadiman NHA, et al. Review on nanocrystalline cellulose in bone tissue engineering applications [J]. Polymers, 2020, 12(12): 2818. DOI: 10.3390/polym12122818
[7] Sun AR, Udduttula A, Li J, et al. Cartilage tissue engineering for obesity-induced osteoarthritis: physiology, challenges, and future prospects [J]. Journal of Orthopaedic Translation, 2021, 26: 3-15. DOI: 10.1016/j.jot.2020.07.004
[8] Yuan N, Rezzadeh KS, Lee JC. Biomimetic scaffolds for osteogenesis [J]. Receptors & Clinical Investigation, 2015, 2(3): 898.
[9] Maturavongsadit P, Narayanan LK, Chansoria P, et al. Cell-Laden nanocellulose/chitosan-based bioinks for 3D bioprinting and enhanced osteogenic cell differentiation [J]. ACS Applied Bio Materials, 2021, 4(3): 2342-2353. DOI: 10.1021/acsabm.0c01108
[10] Meskinfam M, Bertoldi S, Albanese N, et al. Polyurethane foam/nano hydroxyapatite composite as a suitable scaffold for bone tissue regeneration [J]. Materials Science and Engineering: C, 2018, 82: 130-140. DOI: 10.1016/j.msec.2017.08.064
[11] Levengood SKL, Zhang M. Chitosan-based scaffolds for bone tissue engineering [J]. Journal of Materials Chemistry B, 2014, 2(21): 3161-3184. DOI: 10.1039/c4tb00027g
[12] Fourie J, Taute F, du Preez L, et al. Chitosan composite biomaterials for bone tissue engineering—a review [J]. Regenerative Engineering and Translational Medicine, 2020, 8: 1-21.
[13] Chen Y, Udduttula A, Xie X, et al. A novel photocrosslinked phosphate functionalized Chitosan-Sr5(PO4)2SiO4 composite hydrogels and in vitro biomineralization, osteogenesis, angiogenesis for bone regeneration application [J]. Composites Part B: Engineering, 2021, 222: 109057. DOI: 10.1016/j.compositesb.2021.109057
[14] Muxika A, Etxabide A, Uranga J, et al. Chitosan as a bioactive polymer: processing, properties and applications [J]. International Journal of Biological Macromolecules, 2017, 105: 1358-1368. DOI: 10.1016/j.ijbiomac.2017.07.087
[15] Philibert T, Lee BH, Fabien N. Current status and new perspectives on chitin and chitosan as functional biopolymers [J]. Applied Biochemistry and Biotechnology, 2017, 181(4): 1314-1337. DOI: 10.1007/s12010-016-2286-2
[16] Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications [J]. Marine Drugs, 2015, 13(3): 1133-1174. DOI: 10.3390/md13031133
[17] Lizardi-Mendoza J, Monal WMA, Valencia FMG. Chemical characteristics and functional properties of chitosan [M]// Chitosan in the preservation of agricultural commodities. Pittsburgh: Academic Press, 2016: 3-31.
[18] Miculescu F, Maidaniuc A, Miculescu M, et al. Synthesis and characterization of jellified composites from bovine bone-derived hydroxyapatite and starch as precursors for robocasting [J]. ACS omega, 2018, 3(1): 1338-1349. DOI: 10.1021/acsomega.7b01855
[19] Reyna-Urrutia V, Estevez M, González-González A, et al. 3D scaffolds of caprolactone/chitosan/polyvinyl alcohol/hydroxyapatite stabilized by physical bonds seeded with swine dental pulp stem cell for bone tissue engineering [J]. Journal of Materials Science: Materials in Medicine, 2022, 33(12): 81. DOI: 10.1007/s10856-022-06702-2
[20] Kim Y, Zharkinbekov Z, Raziyeva K, et al. Chitosan-based biomaterials for tissue regeneration [J]. Pharmaceutics, 2023, 15(3): 807. DOI: 10.3390/pharmaceutics15030807
[21] Teuku R, Nurhanifa A. The role of poly (lactic acid)/chitosan nanocomposites blend in manufacture non-cytotoxic basic bio scaffold [J]. AIP Conference Proceedings, 2023, 1: 2431.
[22] Xu Z, Sun Y, Dai H, et al. Engineered 3D-printed polyvinyl alcohol scaffolds incorporating β-tricalcium phosphate and icariin induce bone regeneration in rat skull defect model [J]. Molecules, 2022, 27(14): 4535. DOI: 10.3390/molecules27144535
[23] Macfarlane E, Seibel MJ, Zhou H. Arthritis and the role of endogenous glucocorticoids [J]. Bone research, 2020, 8(1): 33. DOI: 10.1038/s41413-020-00112-2
[24] Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage [J]. Biomaterials, 2016, 98: 1-22. DOI: 10.1016/j.biomaterials.2016.04.018
[25] Sadeghianmaryan A, Naghieh S, Sardroud HA, et al. Extrusion-based printing of chitosan scaffolds and their in vitro characterization for cartilage tissue engineering [J]. International Journal of Biological Macromolecules, 2020, 164: 3179-3192. DOI: 10.1016/j.ijbiomac.2020.08.180
[26] Yang J, Jing X, Wang Z, et al. In vitro and in vivo study on an injectable glycol chitosan/dibenzaldehyde-terminated polyethylene glycol hydrogel in repairing articular cartilage defects [J]. Frontiers in Bioengineering and Biotechnology, 2021, 9: 607709. DOI: 10.3389/fbioe.2021.607709
[27] Boyer C, Réthoré G, Weiss P, et al. A self-setting hydrogel of silylated chitosan and cellulose for the repair of osteochondral defects: from in vitro characterization to preclinical evaluation in dogs [J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 23. DOI: 10.3389/fbioe.2020.00023
[28] Nazhvani FD, Amirabad LM, Azari A, et al. Effects of in vitro low oxygen tension preconditioning of buccal fat pad stem cells on in vivo articular cartilage tissue repair [J]. Life Sciences, 2021, 280: 119728. DOI: 10.1016/j.lfs.2021.119728
[29] Luo M, Chen M, Bai J, et al. A bionic composite hydrogel with dual regulatory functions for the osteochondral repair [J]. Colloids and Surfaces B: Biointerfaces, 2022, 219: 112821. DOI: 10.1016/j.colsurfb.2022.112821
[30] Li P, Fu L, Liao Z, et al. Chitosan hydrogel/3D-printed poly (ε-caprolactone) hybrid scaffold containing synovial mesenchymal stem cells for cartilage regeneration based on tetrahedral framework nucleic acid recruitment [J]. Biomaterials, 2021, 278: 121131. DOI: 10.1016/j.biomaterials.2021.121131
[31] Houreh AB, Masaeli E, Nasr-Esfahani MH. Chitosan/polycaprolactone multilayer hydrogel: a sustained Kartogenin delivery model for cartilage regeneration [J]. International Journal of Biological Macromolecules, 2021, 177: 589-600. DOI: 10.1016/j.ijbiomac.2021.02.122
[32] Zhou Y, Xu L, Zhang X, et al. Radiation synthesis of gelatin/CM-chitosan/β-tricalcium phosphate composite scaffold for bone tissue engineering [J]. Materials Science and Engineering: C, 2012, 32(4): 994-1000. DOI: 10.1016/j.msec.2012.02.029
[33] Tonda-Turo C, Carmagnola I, Chiappone A, et al. Photocurable chitosan as bioink for cellularized therapies towards personalized scaffold architecture [J]. Bioprinting, 2020, 18: e00082. DOI: 10.1016/j.bprint.2020.e00082
[34] Chen T, Zou Q, Du C, et al. Biodegradable 3D printed HA/CMCS/PDA scaffold for repairing lacunar bone defect [J]. Materials Science and Engineering: C, 2020, 116: 111148. DOI: 10.1016/j.msec.2020.111148
[35] Müller WEG, Tolba E, Schröder HC, et al. A new printable and durable N,O-carboxymethyl chitosan–Ca2+–polyphosphate complex with morphogenetic activity [J]. Journal of Materials Chemistry B, 2015, 3(8): 1722-1730. DOI: 10.1039/C4TB01586J
[36] Yang Y, Yang S, Wang Y, et al. Anti-infective efficacy, cytocompatibility and biocompatibility of a 3D-printed osteoconductive composite scaffold functionalized with quaternized chitosan [J]. Acta Biomaterialia, 2016, 46: 112-128. DOI: 10.1016/j.actbio.2016.09.035
[37] Yang Y, Chu L, Yang S, et al. Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models [J]. Acta Biomaterialia, 2018, 79: 265-275. DOI: 10.1016/j.actbio.2018.08.015
[38] Kai H, Wang X, Madhukar KS, et al. Fabrication of a two-level tumor bone repair biomaterial based on a rapid prototyping technique [J]. Biofabrication, 2009, 1(2): 025003. DOI: 10.1088/1758-5082/1/2/025003
[39] Chen S, Lau P, Lei M, et al. Segmental composite porous scaffolds with either osteogenesis or anti-bone resorption properties tested in a rabbit ulna defect model [J]. Journal of Tissue Engineering and Regenerative Medicine, 2017, 11(1): 34-43. DOI: 10.1002/term.1828
[40] Cui ZK, Kim S, Baljon JJ, et al. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering [J]. Nature Communications, 2019, 10(1): 1-10. DOI: 10.1038/s41467-018-07882-8
[41] Jafari H, Atlasi Z, Mahdavinia GR, et al. Magnetic κ-carrageenan/chitosan/montmorillonite nanocomposite hydrogels with controlled sunitinib release [J]. Materials Science and Engineering: C, 2021, 124: 112042. DOI: 10.1016/j.msec.2021.112042
[42] Elhefian EA, Nasef MM, Yahaya AH. Preparation and characterization of chitosan/agar blended films: part 2. Thermal, mechanical, and surface properties [J]. Journal of Chemistry, 2012, 9(2): 510-516. DOI: 10.1155/2012/285318
[43] Iqbal DN, Tariq M, Khan SM, et al. Synthesis and characterization of chitosan and guar gum based ternary blends with polyvinyl alcohol [J]. International Journal of Biological Macromolecules, 2020, 143: 546-554. DOI: 10.1016/j.ijbiomac.2019.12.043
[44] Wang W, Meng Q, Li Q, et al. Chitosan derivatives and their application in biomedicine [J]. International Journal of Molecular Sciences, 2020, 21(2): 487. DOI: 10.3390/ijms21020487
[45] Shalumon K, Binulal N, Selvamurugan N, et al. Electrospinning of carboxymethyl chitin/poly (vinyl alcohol) nanofibrous scaffolds for tissue engineering applications [J]. Carbohydrate Polymers, 2009, 77(4): 863-869. DOI: 10.1016/j.carbpol.2009.03.009
[46] Mishra D, Bhunia B, Banerjee I, et al. Enzymatically crosslinked carboxymethyl–chitosan/gelatin/nano-hydroxyapatite injectable gels for in situ bone tissue engineering application [J]. Materials Science and Engineering: C, 2011, 31(7): 1295-1304. DOI: 10.1016/j.msec.2011.04.007
[47] Bukzem AL, Signini R, Dos Santos DM, et al. Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function [J]. International Journal of Biological Macromolecules, 2016, 85: 615-624. DOI: 10.1016/j.ijbiomac.2016.01.017
[48] Bhatia SK, Ramadurai KW. 3D printing and bio-based materials in global health [M]. Switzerland: Springer International Publishing AG, 2017.
[49] Huang GQ, Cheng LY, Xiao JX, et al. Preparation and characterization of O-carboxymethyl chitosan–sodium alginate polyelectrolyte complexes [J]. Colloid and Polymer Science, 2015, 293(2): 401-407. DOI: 10.1007/s00396-014-3432-4
[50] Freitas ED, Moura Jr CF, Kerwald J, et al. An overview of current knowledge on the properties, synthesis and applications of quaternary chitosan derivatives [J]. Polymers, 2020, 12(12): 2878. DOI: 10.3390/polym12122878
[51] Andreica BI, Cheng X, Marin L. Quaternary ammonium salts of chitosan. A critical overview on the synthesis and properties generated by quaternization [J]. European Polymer Journal, 2020, 139: 110016. DOI: 10.1016/j.eurpolymj.2020.110016
[52] Wahba SM, Darwish AS, Kamal SM. Ceria-containing uncoated and coated hydroxyapatite-based galantamine nanocomposites for formidable treatment of Alzheimer’s disease in ovariectomized albino-rat model [J]. Materials Science and Engineering: C, 2016, 65: 151-163. DOI: 10.1016/j.msec.2016.04.041
[53] Federer C, Kurpiers M, Bernkop-Schnürch A. Thiolated chitosans: a multi-talented class of polymers for various applications [J]. Biomacromolecules, 2020, 22(1): 24-56.
[54] Lynn A, Yannas I, Bonfield W. Antigenicity and immunogenicity of collagen [J]. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2004, 71(2): 343-354.
[55] Olszta MJ, Cheng X, Jee SS, et al. Bone structure and formation: a new perspective [J]. Materials Science and Engineering: R: Reports, 2007, 58(3-5): 77-116. DOI: 10.1016/j.mser.2007.05.001
[56] Xing W, Tang Y. On mechanical properties of nanocomposite hydrogels: searching for superior properties [J]. Nano Materials Science, 2021.
[57] Hu X, Man Y, Li W, et al. 3D bio-printing of CS/Gel/HA/Gr hybrid osteochondral scaffolds [J]. Polymers, 2019, 11(10): 1601. DOI: 10.3390/polym11101601
[58] Serra I, Fradique R, Vallejo MCdS, et al. Production and characterization of chitosan/gelatin/β-TCP scaffolds for improved bone tissue regeneration [J]. Materials Science and Engineering: C, 2015, 55: 592-604. DOI: 10.1016/j.msec.2015.05.072
[59] Wu Q, Maire M, Lerouge S, et al. 3D printing of microstructured and stretchable chitosan hydrogel for guided cell growth [J]. Advanced Biosystems, 2017, 1(6): 1700058. DOI: 10.1002/adbi.201700058
[60] Wang J, Yang M, Zhu Y, et al. Phage nanofibers induce vascularized osteogenesis in 3D printed bone scaffolds [J]. Advanced materials, 2014, 26(29): 4961-4966. DOI: 10.1002/adma.201400154
[61] Timashev P, Bardakova K, Demina Т, et al. Novel biocompatible material based on solid-state modified chitosan for laser stereolithography [J]. Современные технологии в медицине, 2015, 7(3): 20-29.
[62] Tsai CH, Hung CH, Kuo CN, et al. Improved bioactivity of 3D printed porous titanium alloy scaffold with chitosan/magnesium-calcium silicate composite for orthopaedic applications [J]. Materials, 2019, 12(2): 203. DOI: 10.3390/ma12020203
[63] Dadhich P, Das B, Pal P, et al. A simple approach for an eggshell-based 3D-printed osteoinductive multiphasic calcium phosphate scaffold [J]. ACS Applied Materials & Interfaces, 2016, 8(19): 11910-11924.
[64] Chen S, Shi Y, Zhang X, et al. Biomimetic synthesis of Mg-substituted hydroxyapatite nanocomposites and three-dimensional printing of composite scaffolds for bone regeneration [J]. Journal of Biomedical Materials Research Part A, 2019, 107(11): 2512-2521. DOI: 10.1002/jbm.a.36757
[65] Haberstroh K, Ritter K, Kuschnierz J, et al. Bone repair by cell-seeded 3D-bioplotted composite scaffolds made of collagen treated tricalciumphosphate or tricalciumphosphate-chitosan-collagen hydrogel or PLGA in ovine critical-sized calvarial defects [J]. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2010, 93(2): 520-530.
[66] Yin X, Li Y, Chen Y, et al. IL-4-loaded alginate/chitosan multilayer films for promoting angiogenesis through both direct and indirect means [J]. International Journal of Biological Macromolecules, 2023, 232: 123486. DOI: 10.1016/j.ijbiomac.2023.123486
[67] Su F, Wang Y, Liu X, et al. Biocompatibility and in vivo degradation of chitosan based hydrogels as potential drug carrier [J]. Journal of Biomaterials Science, Polymer Edition, 2018, 29(13): 1515-1528. DOI: 10.1080/09205063.2017.1412244
[68] Suo H, Zhang J, Xu M, et al. Low-temperature 3D printing of collagen and chitosan composite for tissue engineering [J]. Materials Science and Engineering: C, 2021, 123: 111963. DOI: 10.1016/j.msec.2021.111963
[69] Huang J, Fu H, Wang Z, et al. BMSCs-laden gelatin/sodium alginate/carboxymethyl chitosan hydrogel for 3D bioprinting [J]. RSC Advances, 2016, 6(110): 108423-108430. DOI: 10.1039/C6RA24231F
[70] Vo TS, Kim SK. Potential anti-HIV agents from marine resources: an overview [J]. Marine drugs, 2010, 8(12): 2871-2892. DOI: 10.3390/md8122871
[71] Sukul M, Sahariah P, Lauzon HL, et al. In vitro biological response of human osteoblasts in 3D chitosan sponges with controlled degree of deacetylation and molecular weight [J]. Carbohydrate Polymers, 2021, 254: 117434. DOI: 10.1016/j.carbpol.2020.117434
[72] Pan H, Yang Q, Huang G, et al. Hypolipidemic effects of chitosan and its derivatives in hyperlipidemic rats induced by a high-fat diet [J]. Food & Nutrition Research, 2016, 60(1): 31137.
[73] Patel DK, Dutta SD, Hexiu J, et al. 3D-printable chitosan/silk fibroin/cellulose nanoparticle scaffolds for bone regeneration via M2 macrophage polarization [J]. Carbohydrate Polymers, 2022, 281: 119077.
[74] Hu J, Wang Z, Miszuk JM, et al. Vanillin-bioglass cross-linked 3D porous chitosan scaffolds with strong osteopromotive and antibacterial abilities for bone tissue engineering [J]. Carbohydrate Polymers, 2021, 271: 118440. DOI: 10.1016/j.carbpol.2021.118440
[75] Ma P, Wu W, Wei Y, et al. Biomimetic gelatin/chitosan/polyvinyl alcohol/nano-hydroxyapatite scaffolds for bone tissue engineering [J]. Materials & Design, 2021, 109865.
[76] Maharjan B, Park J, Kaliannagounder VK, et al. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering [J]. Carbohydrate Polymers, 2021, 251: 117023. DOI: 10.1016/j.carbpol.2020.117023
[77] Jiang Z, Zhang K, Du L, et al. Construction of chitosan scaffolds with controllable microchannel for tissue engineering and regenerative medicine [J]. Materials Science and Engineering: C, 2021, 126: 112178. DOI: 10.1016/j.msec.2021.112178
[78] Lee CM, Yang SW, Jung SC, et al. Oxygen plasma treatment on 3D-printed chitosan/gelatin/hydroxyapatite scaffolds for bone tissue engineering [J]. Journal of Nanoscience and Nanotechnology, 2017, 17(4): 2747-2750. DOI: 10.1166/jnn.2017.13337
[79] Aydogdu MO, Oner ET, Ekren N, et al. Comparative characterization of the hydrogel added PLA/β-TCP scaffolds produced by 3D bioprinting [J]. Bioprinting, 2019, 13: e00046. DOI: 10.1016/j.bprint.2019.e00046
[80] Ye X, Li L, Lin Z, et al. Integrating 3D-printed PHBV/Calcium sulfate hemihydrate scaffold and chitosan hydrogel for enhanced osteogenic property [J]. Carbohydrate Polymers, 2018, 202: 106-114. DOI: 10.1016/j.carbpol.2018.08.117
[81] Deng N, Sun J, Li Y, et al. Experimental study of rhBMP-2 chitosan nano-sustained release carrier-loaded PLGA/nHA scaffolds to construct mandibular tissue-engineered bone [J]. Archives of Oral Biology, 2019, 102: 16-25. DOI: 10.1016/j.archoralbio.2019.03.023
[82] Dong L, Wang SJ, Zhao XR, et al. 3D-printed poly (ε-caprolactone) scaffold integrated with cell-laden chitosan hydrogels for bone tissue engineering [J]. Scientific Reports, 2017, 7(1): 1-9. DOI: 10.1038/s41598-016-0028-x
[83] Zhang J, Chen Y, Xu J, et al. Tissue engineering using 3D printed nano-bioactive glass loaded with NELL1 gene for repairing alveolar bone defects [J]. Regenerative Biomaterials, 2018, 5(4): 213-220. DOI: 10.1093/rb/rby015
[84] Lu H, Pan X, Hu M, et al. Fabrication of graphene/gelatin/chitosan/tricalcium phosphate 3D printed scaffolds for bone tissue regeneration applications [J]. Applied Nanoscience, 2021, 11(2): 335-346. DOI: 10.1007/s13204-020-01615-4
[85] Zafeiris K, Brasinika D, Karatza A, et al. Additive manufacturing of hydroxyapatite–chitosan–genipin composite scaffolds for bone tissue engineering applications [J]. Materials Science and Engineering: C, 2021, 119: 111639. DOI: 10.1016/j.msec.2020.111639
[86] Ergul NM, Unal S, Kartal I, et al. 3D printing of chitosan/poly (vinyl alcohol) hydrogel containing synthesized hydroxyapatite scaffolds for hard-tissue engineering [J]. Polymer Testing, 2019, 79: 106006. DOI: 10.1016/j.polymertesting.2019.106006
[87] Wers E, Oudadesse H, Lefeuvre B, et al. Evaluation of the kinetic and relaxation time of gentamicin sulfate released from hybrid biomaterial bioglass-chitosan scaffolds [J]. Applied Surface Science, 2015, 353: 200-208. DOI: 10.1016/j.apsusc.2015.06.146
[88] Shokri S, Movahedi B, Rafieinia M, et al. A new approach to fabrication of Cs/BG/CNT nanocomposite scaffold towards bone tissue engineering and evaluation of its properties [J]. Applied Surface Science, 2015, 357: 1758-1764. DOI: 10.1016/j.apsusc.2015.10.048
[89] Nazemi K, Azadpour P, Moztarzadeh F, et al. Tissue-engineered chitosan/bioactive glass bone scaffolds integrated with PLGA nanoparticles: a therapeutic design for on-demand drug delivery [J]. Materials Letters, 2015, 138: 16-20. DOI: 10.1016/j.matlet.2014.09.086
[90] Puvaneswary S, Talebian S, Raghavendran HB, et al. Fabrication and in vitro biological activity of βTCP-chitosan-fucoidan composite for bone tissue engineering [J]. Carbohydrate Polymers, 2015, 134: 799-807. DOI: 10.1016/j.carbpol.2015.07.098
[91] Reddy N, Santosh MS, Venkatesh K, et al. Alkali treated 3D chitosan scaffolds with enhanced strength and stability [J]. Journal of Polymers and the Environment, 2021, 29: 3302-3310.
[92] Kurian M, Stevens R, McGrath KM. Towards the development of artificial bone grafts: combining synthetic biomineralisation with 3D printing [J]. Journal of Functional Biomaterials, 2019, 10(1): 12. DOI: 10.3390/jfb10010012
[93] Shive MS, Stanish WD, McCormack R, et al. BST-CarGel® treatment maintains cartilage repair superiority over microfracture at 5 years in a multicenter randomized controlled trial [J]. Cartilage, 2015, 6(2): 62-72. DOI: 10.1177/1947603514562064
[94] Gholap AD, Rojekar S, Kapare HS, et al. Chitosan scaffolds: expanding horizons in biomedical applications [J]. Carbohydrate Polymers, 2023: 121394.



 下载:
下载: